Ancient humans and their extinct relatives were exposed to toxic lead for nearly two million years, and this environmental stressor may have shaped the evolution of language and brain development in ways that gave modern humans a decisive advantage. A new study published October 15, 2025, in Science Advances reveals that a single genetic mutation protected Homo sapiens from lead’s neurotoxic effects while Neanderthals remained vulnerable.
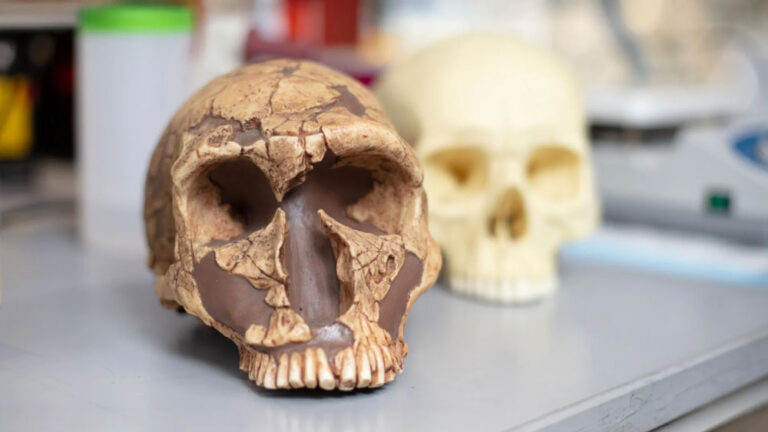
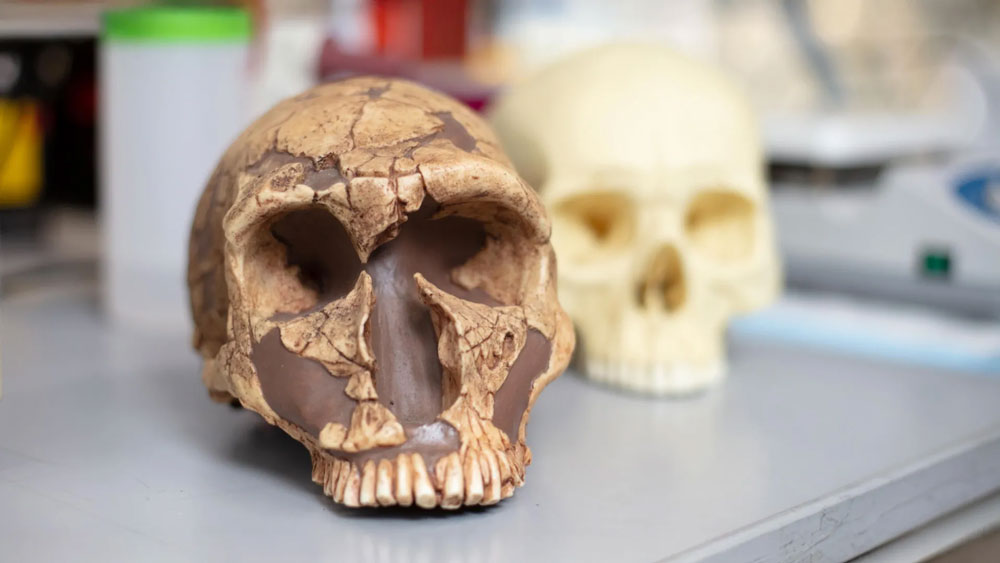
An international research team led by Alysson Muotri from the University of California San Diego and colleagues analyzed 51 fossilized teeth from modern humans, Neanderthals, extinct human ancestors like Australopithecus africanus, and ancient great apes including Gigantopithecus blacki. The specimens, spanning 100,000 to 1.8 million years ago, came from sites across Africa, Asia, and Europe. Using high-precision laser ablation geochemistry, scientists detected lead in 73 percent of the samples, including 71 percent of modern and archaic human teeth.
The findings overturn assumptions that harmful lead exposure began only with Roman plumbing or industrial pollution. Tooth enamel records environmental exposure in growth layers similar to tree rings, capturing episodes of contact with the metal during childhood development. Surprisingly, prehistoric teeth showed lead exposure patterns comparable to individuals born between the 1940s and 1970s, when leaded gasoline and paint contaminated modern environments.
Unlike industrial contamination, prehistoric lead came from natural sources. Volcanic dust, mineral-rich groundwater in caves, and soil containing lead deposits exposed ancient populations episodically throughout their lives. The research team hypothesizes that caves played a central role in this exposure.
“One possibility is that they were looking for caves with running water inside,” said Muotri, professor of pediatrics and cellular and molecular medicine at UC San Diego School of Medicine. “Caves contain lead, so they were all contaminated. Based on the tooth enamel studies, it started very early in infancy.”
Gigantopithecus blacki fossils dating back 1.8 million years showed the most frequent acute lead exposure among all specimens examined. This giant extinct ape sought shelter and water in cave systems where mineralized rocks leached lead into water sources. The banded patterns in tooth enamel reveal that exposure was episodic rather than continuous, reflecting seasonal searches for water and migrations through geologically diverse terrain.
Lead severely impairs developing brains, causing deficits in cognition, emotional regulation, and communication. These effects raise a critical question: how did modern human brains flourish despite chronic lead exposure during evolution while Neanderthals and other relatives disappeared?
The answer appears to lie in a gene called NOVA1, which regulates brain development and neural communication. This master regulator of neurodevelopment controls how neural progenitor cells respond to environmental stressors. Most modern humans carry a variant of NOVA1 that differs by just one DNA base pair from the ancestral version present in Neanderthals and Denisovans.
Previous work by Muotri’s team showed that replacing the human NOVA1 variant with the archaic version in laboratory-grown brain organoids caused significant changes to neural architecture and synaptic connectivity. The archaic variant accelerated early brain maturation but resulted in less network complexity over time.
“If all humans have this newer mutation in all corners of the world, very strong genetic pressure must have selected for it in our species,” Muotri explained.
To test whether lead exposure influenced this selection, researchers created brain organoids with both human and archaic NOVA1 variants and exposed them to lead. They compared the development of cortical and thalamic neurons in both groups. The results proved striking.
Lead exposure altered NOVA1 expression in both variants, affecting genes linked to neurodevelopmental disorders including autism and epilepsy. However, only the archaic NOVA1 variant disrupted expression of FOXP2, a gene essential for speech and language development. People with certain FOXP2 mutations cannot produce sophisticated language.
“These type of neurons related to complex language are susceptible to death in the archaic version of NOVA1,” Muotri noted. “The FOXP2 gene is identical between us and the Neanderthals, but it’s how the gene is regulated by NOVA1 that likely contributes to language differences.”
Organoids carrying the modern human NOVA1 variant maintained healthy brain cell growth under lead exposure, while those with the archaic variant experienced developmental disruptions. This genetic advantage may have allowed language and complex communication to flourish in Homo sapiens despite environmental contamination.
The timing matters. Early Maya lunar observations date back to at least 361 CE, meaning populations had accumulated sufficient data within a century to calibrate predictive systems. Similarly, if the NOVA1 mutation emerged early in human evolution, each generation would have benefited from slightly better protection against episodic lead exposure during critical periods of brain development.
Enhanced communication likely improved social cohesion, cooperation, and cultural transmission in modern human populations. These advantages helped Homo sapiens survive environmental stress and spread globally while Neanderthal populations declined.
“Language is such an important advantage, it’s transformational, it is our superpower,” said Muotri. “Because we have language, we are able to organize society and exchange ideas, allowing us to coordinate large movements. There is no evidence that Neanderthals could do that. They might have had abstract thinking, but they could not translate that to each other.”
Archaeological evidence shows Neanderthals possessed sophisticated tool-making skills, used pigments, and engaged in potential symbolic behavior. The new hypothesis shifts focus from cognitive capacity to neural network resilience under real environmental pressures. If modern humans possessed regulatory protection against lead, even slight differences in communication efficiency could have become crucial during social competition, territorial expansion, and knowledge exchange across greater distances.
Muotri speculates that lead exposure may have contributed to Neanderthal extinction approximately 40,000 years ago, though he emphasizes the hypothesis requires further testing. The study relies on experimental evidence from organoids and chemical analysis of fossils rather than actual ancient DNA, which rarely preserves well enough for detailed genetic analysis.
The research has implications beyond evolutionary history. Understanding how NOVA1 variants modulate sensitivity to environmental toxins could inform preventive strategies in communities where lead persists in aging infrastructure. The findings also illuminate neurological conditions related to language development, including speech apraxia and autism spectrum disorders.
The convergence of tooth enamel analysis and organoid experiments represents a new approach to evolutionary neuroscience. Rather than searching for single “language genes,” researchers now investigate regulatory networks and their interactions with real environmental pressures that ancient populations encountered. Such frameworks help explain how subtle differences in gene regulation could amplify effects of ubiquitous toxins and channel cognitive system development along different trajectories.
The study demonstrates that what set modern humans apart was not simply superior intelligence but a biological infrastructure more resilient to environmental disruption. A deadly metal that once threatened our ancestors may have inadvertently pushed evolution toward favoring a more communicative, adaptive species capable of building complex societies.
Featured image: UC San Diego researchers have found high levels of lead in the teeth of both Neanderthals (left) and modern humans (right). However, a gene mutation may have protected modern human brains, allowing language to flourish. Credit: Kyle Dykes/UC San Diego Health Sciences